Sarcopenia
Sarcopenia is an age-related gradual loss of muscle mass, leading to reduced muscular function and increased physical disability and mortality risk [1, 2]. This condition is estimated to affect between 10 and 20% of the European population, a number that is expected to steadily increase with the gradually ageing population [3]. Currently, the most promising remedy for preventing and treating sarcopenia is physical activity, resistance exercise in particular [4]. Yet, the dosage of exercise that is required to achieve optimal benefits remains unknown. Furthermore, several investigations reports that aging is associated with reduced abilities to benefit from resistance training [5]. This issue is likely due to a decreased sensitivity to the exercise stimulus, and does not seem to be caused by impaired induction of systemic anabolic physiological cues) [6]. The resulting anabolic resistance is associated with reduced acute activation of muscular molecular transducers in muscle that are principal for controlling protein accretion and growth in response to resistance training, including reduced ribosomal biogenesis/accumulation [7].
Mitigation of age-related anabolic resistance through modification of training prescription promise to offer an efficient route for optimizing non-pharmacological therapies to improve health and quality of life across the life span. Currently, training volume is regarded as the primary training variable that governs training responses [8]. As such, manipulating training volume holds the greatest potential for optimizing responses to resistance training. Indeed, in young individuals, 12 weeks of resistance training with moderate volume is associated with more pronounced increases in muscle growth and strength compared to training with low volume [9]. Despite this, ~50 % of young individuals still fail to exhibit beneficial effects of increased training volume, a phenomenon that coincides with impaired abilities to accumulate ribosomes during the early phases of training [9, 10]. In elderly, the benefits of higher training volume promises to be even more decisive, as the anabolic response to acute training with elevated volume is associated with more pronounced augmentation in muscular protein synthesis and activation of key cellular signaling pathways than is observed in the young [6]. However, to date, the long-term responses to elevated training volumes in this age group remains largely unknown. Interestingly, in young individuals, a gradual accumulation of ribosomal entities can be seen during the initial phase of a resistance training intervention, peaking after 6-8 training sessions, presumably acting to precede and prepare the tissue for subsequent growth. In elderly, such ribosomal accumulation seems to be impaired, at least after prolonged training [7, 11], with no study having investigated early-stage accumulation. Overall, current observations indicate that the aging muscle needs a more significant stimulus in terms of training volume to overcome any inhibitory mechanisms. Still, the effects of prolonged resistance training with different volumes on accretion of muscle mass and strength among elderly are not known, and we do not know its effects on ribosomal accumulation or the association between gross muscular responses and ribosomal characteristics.
Mechanistically, age-related anabolic resistance could be related to the low-grade inflammation that accompanies advancing age [12]. As an example, ribosome biogenesis is markedly impaired in conditions with a marked inflammatory phenotype, such as chronic kidney disease [13]. This impairment has been associated with epigenetic modifications of the ribosomal gene promoter [13]. The interaction between age, blood characteristics such as inflammatory status, resistance training, training volume, ribosomal gene transcription/epigenetics and muscle growth remains unknown. The current study aims to provide novel insight into the effects of 12 weeks of resistance training with different training volumes on a range of functional and muscle biological outcome measures in young and elderly. In addition, it aims to provide novel insight into the effects of resistance training on a range of health-related variables, including glucose tolerance, health-related biomarkers in fasted-state blood, markers of the metabolic syndrome and cardiovascular health, all of which are associated with age-related issues [14, 15], and for which we have scarce or conflicting knowledge [16, 17]. For example, while we know that different variants of low-density lipoproteins (LDL) phenotypes convey different atherogenic risk profiles (i.e. LDL is not “just” LDL) [18], we have scarce knowledge about the interaction between resistance training and these phenotypes. Overall, the study will provide insight with direct practical value for improving health and functionality in a lifelong perspective, as well as insight into biological responses to resistance training (and individual variation thereof) that may prove vital for identifying molecular targets for future clinical treatment.
Methodology
Participants
For overview of the intervention, see Figure 1. For flow chart and eligibility criteria, see Figure 2. Thirty young (20-30 years of age) and thirty elderly (70 years of age+) will be recruited to the study. Participants will be recruited through local and social media, which has proven to be a successful for recruiting healthy young and elderly subjects in past projects [9, 19]. In addition, 10 young (20-30 years of age) and 10 elderly (70 years of age+) will be recruited as a reference group.
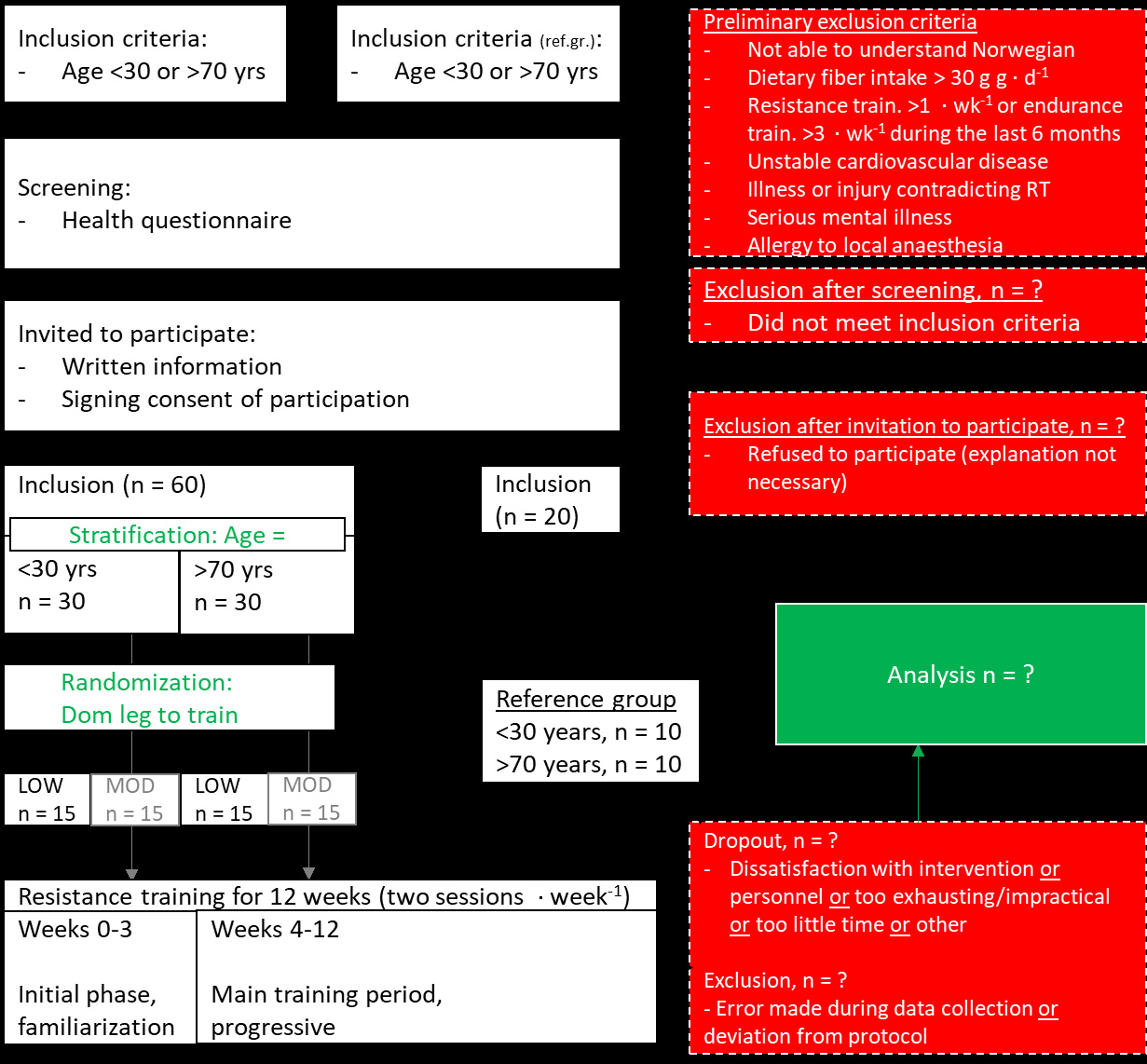
Study overview
The study will be conducted as a 12-week resistance training intervention. All participants will perform the same whole-body training program. Leg and arm exercises will be conducted in a unilateral manner, with one leg/arm performing low-volume training and the other leg/arm performing moderate-volume training. Effects of the intervention on health variables and muscle mass and strength will be investigated at three time points: pre-intervention (0 weeks), after 6 weeks of training and post-intervention (12 weeks) (see Figure 1). Effects of the intervention on muscle biological characteristics will be investigated at three time points: pre-intervention (Week 0), after the sixth training session (Week 3), and post-intervention (Week 12) (see Figure 1). These time points thus coincide with testing and blood/biopsy sampling. At pre- and post-intervention, these test procedures will be conducted during three separate days. At week 3 and 6, they will be conducted during one singular day. All procedures are established at the laboratory facilities at Inland Norway University of Applied Sciences, campus Lillehammer. Prior to onset of the intervention, the study protocol and outcome measures will be preregistered at clinicaltrials.gov. The intervention will be performed in accordance with the declaration of Helsinki.
Resistance training protocol
Resistance training will be performed using a whole-body training program, consisting of two weekly training sessions, each containing three lower-body exercises (leg press, knee extension and - flexion) and three upper-body exercises (chest-press, rows and bicep curls). All exercises will be performed in a unilateral manner, with one leg/arm conducting low-volume training (1x 10RM per exercise) and the other leg/arm conducting moderate-volume training (3x 10RM per exercise), allocated in a randomized manner. Before each training session, participants will register their subjectively perceived bodily feeling (1-5 scale). After lower-body exercises and after upper-body exercises, participants will register their perceived rate of exertion using a 10-point scale. Trained personnel will monitor all sessions. Training volume and loads will be logged.
Sampling of muscle tissue and blood and analyses
Muscle biopsies will be collected from m. vastus lateralis at three timepoints during the intervention (Figure 1): i) pre-intervention (Week 0), ii) after six training sessions (Week 3), and iii) post-intervention (Week 12). All biopsies will be sampled in an overnightfasted condition at the same time of day. Biopsies will be sampled under antiseptic and anesthetized conditions (Lidokain 10 mg ml-1, Mylan Hospital AS, Oslo Norway), using the well-established, minimally invasive micro-biopsy technique [9, 19], using a 12-14 gauge needle (Universal Plus, Mermaid medical A/S, Stenløse, Denmark) operated with a spring loaded biopsy gun (Bard Magnum, Bard Norway A/S, Oslo, Norway). Subsequent to muscle sampling, biopsies will be divided into aliquots for determination of total RNA/targeted mRNA abundances (qPCR/RNA-seq), protein abundances (Western blotting/Mass spec), genome characteristics such as nucleotide, DNA methylation/histone modifications and rDNA copy number (qPCR/bisulphite qPCR/sequencing), metabolimics/lipidomics (mass spec), and muscle fiber characteristics (immunohistochemistry). Blood will be collected at three timepoints during the intervention (Figure 1): i) pre-intervention (0 weeks), ii) after 3 weeks of training, and iii) post-intervention (12 weeks). At all timepoints, blood will be collected from an antecubital vein in a rested and fasted state using standard blood sampling equipment, performed by experienced personnel. At pre- and post-intervention, blood will also be collected following ingestion of a 75 gram bolus of glucose (30 min, 1h and 2h after intake), with concomitant blood sampling using finger sticks every 15 min for determination of blood glucose levels. Together, these blood samples/finger sticks will enable analyses of the effects of the resistance training intervention on variables such as rested-state hormones, inflammatory markers, lipoprotein profiles and metabolomics/lipidomics, whole blood transcriptomics/DNA methylation/rDNA copy number, as well as its effects on glucose tolerance. Blood analyses will be performed at facilities such as Innlandet Hospital Trust, Oslo University Hospital, Copenhagen University (Denmark) and Nightingale Health Ltd (Finland). Glucose analyses will be performed using in-house equipment.
Measurement of muscle strength
Muscle strength test will be performed at three timepoints during the intervention (Figure 1): i) preintervention (0 weeks), ii) after 6 weeks of training, and iii) post-intervention (12 weeks). These tests will consist of unilateral isokinetic extension torque (leg and arm), isometric extension force (leg and arm), and 1RM leg press and isometric bicep curl. At each test day, isokinetic and isometric measurements will be repeated three times, whereupon the highest value will be carried forward to final analyses. To ensure reliability of muscle strength data, participants will be asked to refrain from training during the 2 days leading up to testing. They will also undergo familiarization to testing prior to preintervention testing.
Measurement of body mass composition
Body mass composition/muscle mass will be measured using dual-energy x-ray absorptiometry (DXA), magnetic resonance imaging (MR) and ultrasound (US) at two or three timepoints during the intervention: ): i) pre-intervention (0 weeks, DXA/MR/US), ii) after 6 weeks of training (DXA/US), and iii) post-intervention (12 weeks, DXA/MR/US). These analyses will be performed using established procedures [9, 19].
Measurement of hemoglobin mass
Hemoglobin will be measured at two timepoints during the intervention: i) pre-intervention (0 weeks) and iii) post-intervention (12 weeks). It will be measured using the well-established COrebreathing method [20].
Assessment of health-related quality of life and activities of daily living
Health-related quality of life and activities of daily living will be assessed at two timepoints during the intervention: i) pre-intervention (0 weeks) and iii) post-intervention (12 weeks). Health-related quality of life will be assessed using a previously established questionnaire (SF-36; e.g. [11]). Activities of daily living will be assessed using the gold-standard accelerometry approach (five days), as well as using an established questionnaire [11].
Ethical considerations
Resistance training and physical testing is not associated with significant risk, especially when it is performed in strength training apparats. Still, injuries may occur. To counteract adverse event, trained personnel will carefully monitor every training session. All participants will be offered postintervention consultation on prospective training programs. Muscle biopsy sampling will be performed by experienced personnel, using well-established procedures. Participants will be excluded from the study if they report or experience adverse effects to local anaesthetics. Participants will be given written and oral information on post-procedure care to minimize the risk of infection. Following sampling of muscle tissue, one may experience mild soreness that usually normalizes during 1-2 days after the procedure. Antecubital blood sampling is not associated with risks. Participants will be given access to their own test data on request. The proposed study will add Version 2, 26.06.2021 basic knowledge to our understanding of optimal applications of study design to investigate responses to training, and mechanisms determining exercise training adaptations. Further understanding these basic mechanisms will aid in designing better studies and better suited exercise programs for a wide range of populations.
Data management, study financing and dissemination
Data management and biobank
The data management plan is in line with the FAIR-principle. The project will be integrated into the Norwegian Services for sensitive data (TSD), allowing collection, storage, sharing and analyses of sensitive research data in a secure environment. All data will remain coded (with volume condition remaining unknown) until after completion of cleaning of primary outcome data. Muscle biopsy material will be transferred to Denmark for analyses of metabolomics/lipidomics. Blood samples will be transferred to Denmark for analyses of metabolomics/lipidomics and to Finland for analyses of lipoprotein profiles (Nightingale Health Ltd). After finalization of these analyses, the biological samples in question will be destroyed. After finalization of the project (31.12.2026), remaining biological samples will be transferred to the general biobank “«The TrainOME – humane cellers tilpasning til trening og miljø» (REK-ID: 2013/2041).
Study financing
The study will be financed by the Inland Norway University of Applied Sciences. The project consortium has no conflicts of interest to declare.
Dissemination
The results will be published in peer-reviewed international biomedical and biological journals, preferably open access. In addition, the results will be presented at scientific conferences (national and international) and will be communicated to the general public through mass media, social media, web blogs and podcasts.
References
- Evans, W.J. and J. Lexell, Human Aging, Muscle Mass, and Fiber Type Composition. The Journals of Gerontology: Series A, 1995. 50A(Special_Issue): p. 11-16.
- Beaudart, C., et al., Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLOS ONE, 2017. 12(1): p. e0169548.
- Ethgen, O., et al., The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action. Calcified Tissue International, 2017. 100(3): p. 229-234.
- Fragala, M.S., et al., Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. The Journal of Strength & Conditioning Research, 2019. 33(8).
- Welle, S., S. Totterman, and C. Thornton, Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci, 1996. 51(6): p. M270-5.
- Kumar, V., et al., Muscle Protein Synthetic Responses to Exercise: Effects of Age, Volume, and Intensity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2012. 67(11): p. 1170-1177.
- Brook, M.S., et al., Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. The Journal of Physiology, 2016. 594(24): p. 7399-7417.
- Ralston, G.W., et al., The Effect of Weekly Set Volume on Strength Gain: A Meta-Analysis. Sports Medicine, 2017. 47(12): p. 2585-2601.
- Hammarström, D., et al., Benefits of higher resistance-training volume are related to ribosome biogenesis. The Journal of Physiology, 2020. 598(3): p. 543-565.
- Hammarström, D., Determinants of intra-individual variation in adaptability to resistance training of different volumes. Vol. http://urn.kb.se/resolve?urn=urn:nbn:se:gih:diva-6691. 2021: PhD dissertation, Gymnastik- och idrottshögskolan (GIH).
- Mølmen, K.S., et al., Vitamin D 3 supplementation does not enhance the effects of resistance training in older adults. Journal of Cachexia, Sarcopenia and Muscle, 2021. https://doi.org/10.1002/jcsm.12688.
- Dalle, S., L. Rossmeislova, and K. Koppo, The Role of Inflammation in Age-Related Sarcopenia. Frontiers in Physiology, 2017. 8.
- Zhang, L., et al., Mechanisms Regulating Muscle Protein Synthesis in CKD. Journal of the American Society of Nephrology, 2020. 31(11): p. 2573-2587.
- Bonomini, F., L.F. Rodella, and R. Rezzani, Metabolic syndrome, aging and involvement of oxidative stress. Aging and disease, 2015. 6(2): p. 109-120.
- North, B.J. and D.A. Sinclair, The Intersection Between Aging and Cardiovascular Disease. Circulation Research, 2012. 110(8): p. 1097-1108.
- Westcott, W.L., Resistance Training is Medicine: Effects of Strength Training on Health. Current Sports Medicine Reports, 2012. 11(4).
- 17. Strasser, B., U. Siebert, and W. Schobersberger, Resistance Training in the Treatment of the Metabolic Syndrome. Sports Medicine, 2010. 40(5): p. 397-415.
- Hirano, T., Pathophysiology of Diabetic Dyslipidemia. Journal of Atherosclerosis and Thrombosis, 2018. advpub.
- Mølmen, K., et al., Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J. Cachexia Sarcopenia Muscle, accepted, Jan 2021. https://doi.org/10.1002/jcsm.12688.
- Siebenmann, C., et al., CORP: The assessment of total hemoglobin mass by carbon monoxide rebreathing. Journal of Applied Physiology, 2017. 123(3): p. 645-654.



